Chemistry 2015 JAMB Past Questions
Chemistry 2015 JAMB Past Questions
1. The filter in the cigarette reduce the nicotine construct by
- A. burning
- B. adsorption
- C. evaporation
- D. absorption
Correct Answer: Option B
Explanation
The acetate and paper modify the particulate smoke phase by particle retention (filtration), and finely divided carbon modifies the gaseous phase (adsorption)
2. Which of these require crystallization most?
- A. Drug making
- B. Cement making
- C. Paint making
- D. Perfume making
Correct Answer: Option A
Explanation
Crystallization is required the most in industries where the purity of their products is important e.g in the manufacture of drugs and in sugar production. Options B,C and D do not undergo crystallization
3. Iron is often galvanized in order to
- A. Make it more malleable
- B. Remove the impurities unit
- C. Protect it against corrosion
- D. Render it passive
Correct Answer: Option C
Explanation
Iron is galvanized with zinc covering to protect it against corrosion. Option A, B, D are the main reasons iron is galvanized
4. In the industrial production of H2 is removed by (solution)
- A. washing under pressure
- B. drying over phosphorus (V) oxide
- C. passing the mixture to the limewater
- D. using ammonical copper (I) chlorine
Correct Answer: Option A
Explanation
Washing under pressure is an action in the industrial production of H. Options B,C,D will not yield Co and H
5. The gas that is most useful in protecting humans against solar marathon is
- A. chlorine
- B. ozone
- C. carbon IV oxide
- D. hydrogen sulphur
Correct Answer: Option B
Explanation
The ozone layer or ozone shield refers to a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet (UV) radiation. It contains high concentrations of ozone (O3) relative to other parts of the atmosphere, although still very small relative to other gases in the stratosphere
6. Vulcanization involve the removal of
- A. monometer
- B. the single bond
- C. the double bond
- D. a polymer
Correct Answer: Option C
Explanation
Vulcanizer is the removal of double bend in a chemical reaction. Option A,B,D can not be removed by vulcanizer
7. The acid in electrolysis of water is dilute
- A. HNO3
- B. CH3COOH
- C. H2SO
- D. HCl
Correct Answer: Option C
Explanation
HeSO4 is the acid used in the electrolysis of water. Option A,B,D are concentrated acid and they will react with the water
8. A small
quantity of solid ammonium chloride (NH4Cl) texted gently in a test
tube, the solid gradually disappear produce two gases. Later, a white
cloudy deposit was absence on the cooler part of the test tube. The
ammonium chloride is to have undergo
- A. distillation
- B. sublimation
- C. precipitation
- D. evaporation
Correct Answer: Option B
Explanation
Sublimation is the transformation of solid gaseous state without passing through the liquid state. Options A,C,D do not show case these character of sublimation as they do not bye pass states. They are in a particular order
9. When salt loses its water of crystallization to the atmosphere on exposure, the process is said to be
- A. efflorescence
- B. déliquescence
- C. effervescence
- D. fluorescence
Correct Answer: Option A
Explanation
Efflorescence is a process of long exposure to the atmosphere which favors salt loss of water. Options B, C, D are not related to loss of water
10. Atomicity of ozone is
- A. 1
- B. 2
- C. 3
- D. 4
Correct Answer: Option C
Explanation
Ozone means Oz as the atomicity is 3. Option A,B,D cannot give ozone because their atomicity is not 3
11. Which of the noble gases has the greatest ionization energy
- A. He
- B. Xe
- C. Ar
- D. Rr
Correct Answer: Option A
Explanation
Helium has the greatest ionization energy because they are less stable. Options B,C,D have low ionization energy because one more stable and do not react easily
12.The weakest attractive force that can be observed between two molecules is
- A. ionic
- B. covalent
- C. co-ordinate covalent
- D. vander Waals
Correct Answer: Option D
Explanation
Vander Waals are the weakest attractive forces that exist between two molecules. Option A,B,C are not weak forces because the force attraction that exist between their interested molecule are very strong
13. An elements used in production of matches is
- A. nitrogen
- B. aluminum
- C. copper
- D. sulphur
Correct Answer: Option D
Explanation
Sulphur is an element that burns with a strongly explosive smell, as a result it is explosive smell
14. Cathode rays
cause an object placed behind a perforated anode to cast a shadow on the
screen. This observation shows that the rays
- A. are positively charged
- B. are negatively charged
- C. Have mass
- D. travel in straight lines
Correct Answer: Option D
Explanation
Rays travels in straight lines and finds its way through any opening available. It could be refracted, reflected or transmitted based on any obstacle. Option A,B,C are not the reason why the cathode are coasted shadows
15.Flow of current in electrolytes is due to the movement of
- A. electrons
- B. Holes and electron
- C. Ions
- D. Charges
Correct Answer: Option C
16. A suitable reagent for distinguish between ethanoic and ethanol is
- A. bromine water
- B. Fehling’s solution
- C. sodium hydrogen trioxocarbonate (iv)
- D. Ammoniacal silver trioxonitrate(V)
Correct Answer: Option C
Explanation
Sodium hydrogen trionocarbonate iv is the only distinguishing reagent between ethanoic and ethanol. Option A, B, D are not conspicuous in distinguishing between ethanoic and ethanol
17. In the discovery of protein, the instrument used is
- A. cathode ray tube
- B. glass tube and discharge tube
- C. discharge tube with terminal cathode
- D. discharge tube with central cathode
Correct Answer: Option D
Explanation
Discharge tube with central cathode is an apparatus for producing cathode rays and positive rays. Option A,B,C will not yield cathode rays and positive rays
18
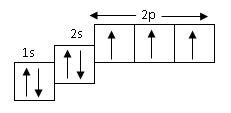
The above orbital diagram shown the electronic configuration of
- A. chlorine
- B. nitrogen
- C. calcium
- D. neon
Correct Answer: Option B
Explanation
Nitrogen has an electronic configuration of 7 i.e1s2 2ps2 2p3. option A,C,D are chlorine 17, calcium 20, neon 10
19. Which of the following metals burns with brick red
- A. Pb
- B. Ca
- C. Na
- D. Mg
Correct Answer: Option B
Explanation
Calcium is the only metal that burn with brick red flame. Option A,C,D will not burn with brick red flame rather burn with brick blue flame
20.In the production of soap, concentrated sodium chloride solution is added to
- A. increase the solubility of soap
- B. decrease the solubility of the soap
- C. saponify the soap
- D. emulsify the soap
Correct Answer: Option B
Explanation
Concentrated sodium chloride solution will the solubility of soap along the course of soap production. Options A,C,D are not appropriate
21. A liquid that will dissolve fat is
- A. hydrochloric acid
- B. calcium hydrochloride
- C. kerosene
- D. water
Correct Answer: Option C
Explanation
Kerosene is only liquid that will dissolve fat. Option A,B,D will not dissolve fat because of their chemical composition
22. Tartaric acid is used industrially to
- A. make baking powder
- B. make fruit juice
- C. remove rust
- D. dry substance
Correct Answer: Option A
Explanation
A is the correct answer. Tartaric acid is found in cream of tartar, which is used in cooking candies and frostings for cakes. Tartaric acid is also found in baking powder, where it serves as the source of acid that reacts with sodium bicarbonate (baking soda)
23. P1V1 = P2V2 supports ?
- A. Charles’s law
- B. Boyles’s law
- C. Graham’s law
- D. Avogadro’s law
Correct Answer: Option B
Explanation
V = 1/Pcross multiply
K = VP
V1P1 = V2V2
For a fixed amount of an ideal gas kept at a fixed temperature, pressure and volume are inversely proportional. Or Boyle's law is a gas law, stating that the pressure and volume of a gas have an inverse relationship, when temperature is held constant.
The equations supports only Boyle’s law. Option A,C,D are not in support of the equation...
24. A fixed mass of gas a volume of 92cm3 at 3°C. When will be its volume at 18C if the pressure remains constant?
- A. 15.3cm3
- B. 87.3cm3
- C. 2.0cm3
- D. 97.0cm3
Correct Answer: Option D
Explanation
=
= 3 + 273 = 276K
= 18 + 273 = 291K
=
= ?
= =
=
25. Which of the following ion’s requires the quantity of electricity for discharge at an electrode
- A. 2.0 mole of Q3+
- B. 2.5 mole of R2+
- C. 3.0 mole of T-
- D. 4.0 mole of Y-
Correct Answer: Option A
Explanation
2.0 mole of Q3+ ions require the largest quantity of electricity for discharge at an electric. option B,C,D are all carrying a smaller quantity of electricity which not be proven for ant discharge



Comments
Post a Comment