Chemistry 2016 JAMB Past Questions
Chemistry 2016 JAMB Past Questions
1.In an electrochemical cell, polarization is caused by
- A. Oxygen
- B. Hydrogen
- C. Tetraoxosulphate(IV) acid
- D. Chlorine
2. The condition required for corrosion to take place is the presence of
- A. Oxygen and Carbon (IV) oxide
- B. Water and Oxygen
- C. Water and Carbon (IV) oxide and Oxygen
- D. Water and Carbon (IV) oxide
3.The enzyme used in the hydrolysis of starch to dextrin and maltose is
- A. Zymase
- B. Sucrase
- C. Amylase
- D. Lactase
4.In the
laboratory preparation of chlorine from concentrated hydrochloric acid
in the presence of potassuim tetraoxomanganate(VII) the produced is
dried by passing it through
- A. Concentrated tetraoxosulphate (VI) acid
- B. Anhydrous calcium chloride
- C. Calcium oxide
- D. Sodium hydroxide
5.The monomer of nylon is
- A. Hydroxybenzene
- B. Hexanedioic acid
- C. Benzene -1,-4-dicarboxylic acid
- D. Ethane-1,2-diol
6. The principle of column chromatography is based on th ability of the constituents to
- A. React with the solvent in the column
- B. React with each other in the solvent in the column
- C. Dissolve in each other in the column
- D. Move at different speeds in the column
7.Ammonium chloride can be separated from its mixture with common salt by
- A. Distillation
- B. Evaporation
- C. Decantation
- D. Sublimation
8. The ability of carbon to form long chains is referred to as
- A. Alkylation
- B. Acylation
- C. Carbonation
- D. Catenation
9.The industrial preparation of hydrogen gas from water gas is referred to as
- A. Bosch process
- B. Contact process
- C. Haber process
- D. Bayer Process
Li is
- A. 6.2
- B. 6.8
- C. 7.1
- D. 6.9
Explanation
( x 7) + ( x 6)= 6.3 + 0.6 = 6.9
11.The colour of litmus in an alkaline medium is
- A. Red
- B. Yellow
- C. Pink
- D. Blue
12. The compounds CH3CH2CHO and CH3COCH3 can be distinguished from each other using
- A. Dilute ammonia
- B. Benedict's solution
- C. Tollen's reagent
- D. Sodium hydroxide solution
13.Which of the following statements is true about 2-methylpropane and butane
- A. They have different number of carbon atoms
- B. They are members of same homologous series
- C. They have the same boiling points
- D. They have the same chemical properties
14.The process that requires the use of hardwater in its operation is
- A. Tanning
- B. Bottling
- C. Laundry
- D. Dyeing
Correct Answer: Option B
16. The radioisotope used in industrial radiography for the rapid checking of faults in welds and casting is
- A. Phosphorus-32
- B. Cobalt-60
- C. Carbon-14
- D. Iodine-131
Correct Answer: Option B
17. The compound produced when sodium peroxide is heated with excess sodium is
- A. Na2CO3
- B. Na2O
- C. NaNO2
- D. NaOH
18. The pollutant that contributes to the depletion of the ozone layer is
- A. CFCs
- B. NO2
- C. SO2
- D. CO
19. Which of the following properties is not peculiar to matter?
- A. Kinetic energy of particles increases from solid to gas
- B. Random motion of particles increases from gas to solid
- C. Orderliness of particles increases from gas to liquid
- D. Random motion of particles increases from liquid to gas
20. Cast iron is used in making
- A. Chains and agricultural implements
- B. Iron sheets and retort stand
- C. Nails and iron rods
- D. Stoves and cookers
21. Which of the following compounds is used as a gaseous fuel?
- A. CH3 -CH2 -CHO2 -COOH
- B. CH3 -C = CH
- C. CH3 -CH2 -CH2 -CH3
- D. CH2 -CH2 -CH3
Correct Answer: Option C
22. The product formed when propanol is heated over a copper catalyst in the absence of oxygen are
- A. CH3CH2CHO and H2
- B. CH3CHO and CO2
- C. CH3CH2COOH and H2
- D. CH3COOH and CO2
Correct Answer: Option D
23. Alkanols have the general molecular formula
- A. C1H2n-2
- B. C1H2n-1CHO
- C. C1H2n-2OH
- D. C2H2n+1OH
Correct Answer: Option D
24. Which of the following is not an alkali?
- A. Ca(OH)2
- B. NH3
- C. NaOH
- D. Al(OH)3
Correct Answer: Option B
Explanation
Both NH3 and Al(OH)3 are not alkali
25. Which of the following statements is correct about the periodic table?
- A. Elements in the same group have the same number of electron shells
- B. The non-metallic properties of the elements tend to decrease across each period
- C. Elements in the same period have the same number of valence electrons
- D. The valence electrons of the elements increase progressively across the period
26. The metal whose ore can be concentrated by passing it through a magnetic separator is
- A. Zn
- B. Sn
- C. Cn
- D. Ca
27. An example of an acidic oxide is
- A. NO
- B. CO
- C. SO2
- D. CuO
Correct Answer: Option C
28. What volume of 0.5M H2SO4 will exactly neutralize 20cm3 of 0.1M NaOH Solution?
- A. 2.0cm 3
- B. 5.0cm 3
- C. 6.8cm 3
- D. 8.3cm 3
Correct Answer: Option A
Molarity of
Volume of
Molarity of
mol NaOH =
⇒
=
Explanation
The balanced equation is given as:Molarity of
Volume of
Molarity of
mol NaOH =
⇒
=
29. Diamond cannot be used
- A. In making bicycle chains
- B. As abrasives
- C. In cutting glass and metals
- D. As dies for drawing wires
30.Ethanol is soluble in water due to the presence of a
- A. Carbonyl group
- B. Hydroxyl groupd
- C. Phenyl group
- D. Methyl group
31. The number of electronic shells contained in an atom with electron configuration 1s22s22p63s23p64s2 is
- A. 3
- B. 2
- C. 4
- D. 5
Correct Answer: Option C
32. The drying agent suitable for drying ammonia is
- A. Calcium Chloride
- B. Calcium Oxide
- C. Phosphorus(V) Oxide
- D. Concentrated H2SO4
Correct Answer: Option B
33.The arrangement of particles in crystal lattices can be studied using
- A. X-rays
- B. -rays
- C. -rays
- D. -rays
- A. PH3 CO and CO2
- B. CO and C
- C. N2 , CO and CO2
- D. PH3 and CO
- A. Blue
- B. Red
- C. Orange
- D. Purple
- A. Butter
- B. Hair cream
- C. Milk
- D. Cod-liver oil
- A. 16
- B. 15
- C. 31
- D. 46
- A. 0.056dm3
- B. 0.224dm3
- C. 224.000dm3
- D. 56.000dm3
- A. Neon
- B. Radon
- C. Xenon
- D. Argon
- A. A reversible reaction
- B. A spontenous reaction
- C. An endothermic reaction
- D. An exothermic reaction
- A. 10H+ (aq)
- B. 5H+ (aq)
- C. 8H+ (aq)
- D. 4H+ (aq)
- A. Geometric isomerism
- B. Positional isomerism
- C. Structural isomerism
- D. Optical isomerism
- A. Condensation and hydrolysis
- B. Fermentation and condensation
- C. Polymerization and hydrolysis
- D. Polymerization and condensation
- A. Alkene and halo-group
- B. Hydroxyl and chloro-group
- C. Alkene and chloro-group
- D. Hydroxyl and halo-group
- A. Enthalphy
- B. Activated complex
- C. Activation energy
- D. Enthalphy change
- A. A spontaneous reaction
- B. An exothermic reaction
- C. A non-spontaneous
- D. An endothermic reaction
- A. Strong acid and strong base
- B. Strong acid and weak base
- C. Weak acid and weak base
- D. Weak acid and strong base
- A. K
- B. M
- C. L
- D. N
- A. Concentration
- B. Catalyst
- C. Temperature
- D. Light
- A. Increase the reactant production
- B. Increase the value of the equilibrium constant
- C. Shift the equilibrium to the left
- D. Decrease the value of the equilibrium constant
Correct Answer: Option A
34. In the extraction of iron, the waste gas from the furnace is a mixture of
Correct Answer: Option C
Explanation
thefrom the hot air blast to burn the coke (48%), the (23%) and (21%) produced from the reduction of the iron ore by carbon (the coke) are the major components of the waste from the extraction.
35. In a neutralization reaction involving HCI and NaOH using litmus as indicator, the colour at the end point is
Correct Answer: Option D
Explanation
Neutral litmus paper is purple. the result of a neutralization reaction is salt and water and hence is neutral.
36. An example of a solid emulsion is
Correct Answer: Option A
37. An isotope has an atomic number of 15 and a mass number of 31. The number of proton it contains is
Correct Answer: Option B
38. Calculate the volume in cm3 of oxygen evolved at s.t.p when a current of 5A is passed through acidified water for 193s?
[F = 96500 C mol1. Molar volume of a gas at s.t.p = 22.4dm3]
Correct Answer: Option A
40H 2H2O + O2 + 4e
4 x 96500C = 22.4dm3
965C = x
x =
= 0.056dm3
Explanation
Q = 193 x 5 = 965C40H 2H2O + O2 + 4e
4 x 96500C = 22.4dm3
965C = x
x =
= 0.056dm3
39. The gas that is used for the treatment of cancer is
Correct Answer: Option B
40. A chemical reaction in which the hydration energy is greater than the lattice energy is referred to as
Correct Answer: Option D
41.
MnO4(aq) + Y 5Fe2+ (aq)
Mn2+ (aq) + 5Fe 2+ (aq) + 4H2O(1)
In the equation above, Y is
In the equation above, Y is
Correct Answer: Option C
42
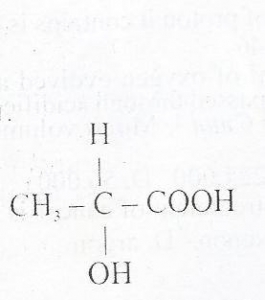
The compound above exhibits
Correct Answer: Option D
43
n monosaccharide polysaccharide -n water.
In the process above, P and Q respectively represent
In the process above, P and Q respectively represent
Correct Answer: Option A
44
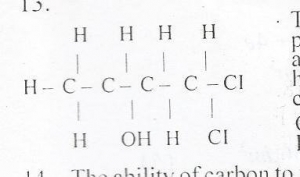
The functional groups present in the compound above are
Correct Answer: Option B
45
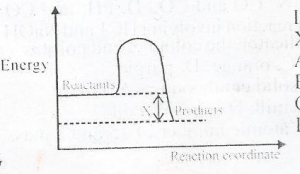
In the diagram above, X is the
Correct Answer: Option D
46

The diagram represents
Correct Answer: Option C
47
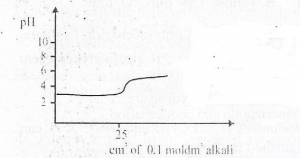
The curve depicts titration between
Correct Answer: Option C
48
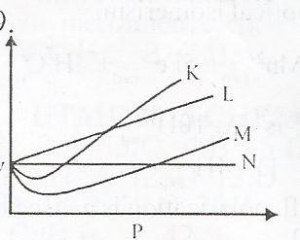
From the diagram above, an ideal gas can be represented by
Correct Answer: Option D
49. CH4(g) + CI2(g) CH2CI(s) + HCIg
The major factor that influences the rate of the reaction above is
Correct Answer: Option D
50. N2O4(aq) 2NO2(g) H = +ve
In the reaction above, an increase in temperature will
Correct Answer: Option B



Comments
Post a Comment